iPS Cell Therapy for Parkinson's Disease: A Safe and Effective Clinical Trial
2025-04-22
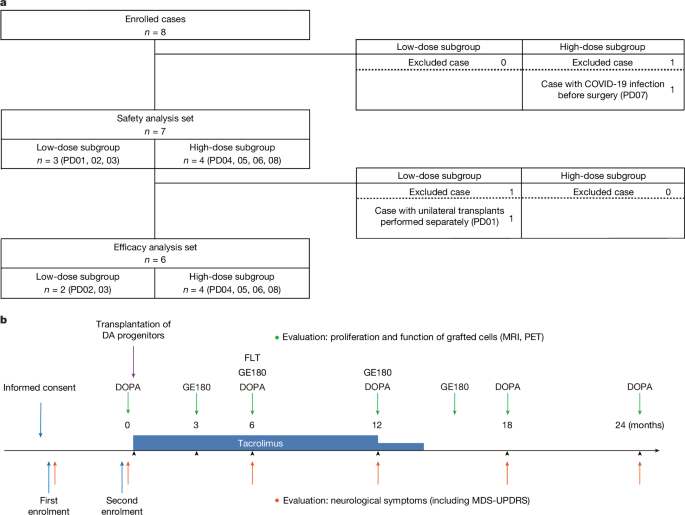
A clinical trial for Parkinson's disease used induced pluripotent stem cell (iPS cell)-derived dopamine progenitor cells in bilateral putaminal transplantation. Results showed the therapy to be safe and effective, with no serious adverse events and improvements in motor symptoms and increased dopamine uptake in some patients. While limitations exist, including potential placebo effects and observer bias, and further research is needed to define optimal patient selection criteria, the trial provides evidence for the safety and efficacy of iPS cell-derived dopamine progenitor cells as a regenerative therapy for Parkinson's disease.
Tech
regenerative therapy